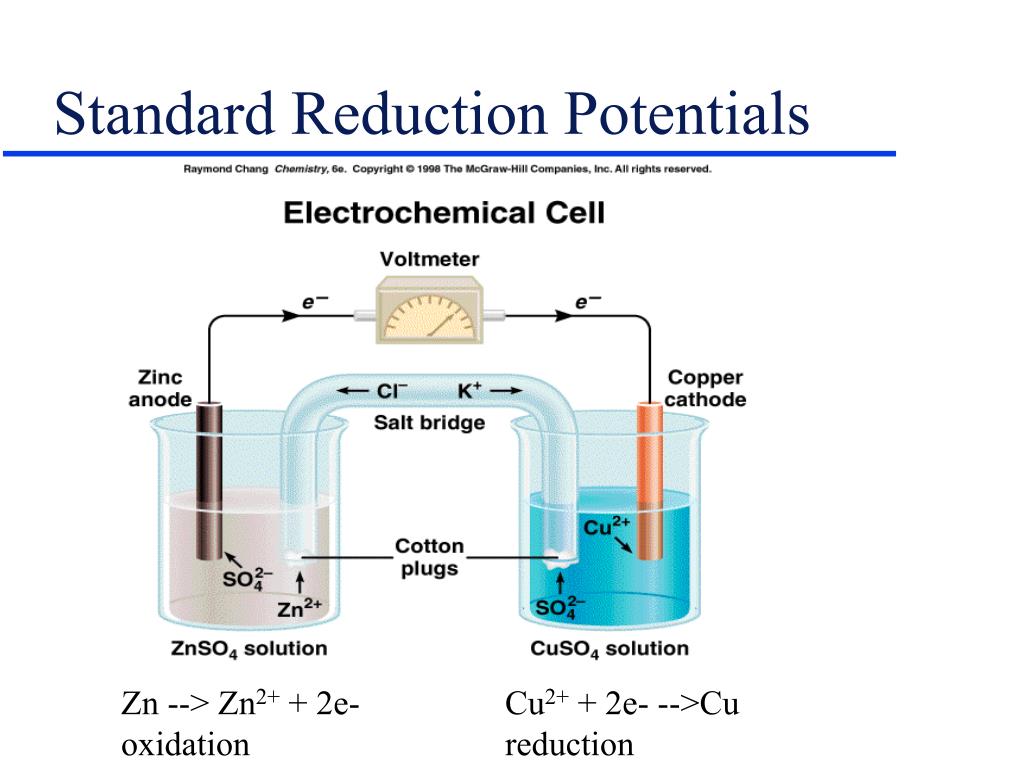
Standard Reduction Potential Zn . reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard reduction potentials by value. The following table provides eo for selected reduction. You may rewrite a reaction by. the she is rather dangerous and rarely used in the laboratory. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The overall cell potential is the reduction. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows standard apparent reduction potentials in biochemistry at ph 7. Its main significance is that it established the zero for standard.
from www.slideserve.com
The overall cell potential is the reduction. 372 rows standard apparent reduction potentials in biochemistry at ph 7. The following table provides eo for selected reduction. Standard reduction potentials by value. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. 45 rows standard electrode potentials in aqueous solution at 25°c. reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by. Its main significance is that it established the zero for standard. the she is rather dangerous and rarely used in the laboratory.
PPT Dr. Marc Madou PowerPoint Presentation, free download ID6718664
Standard Reduction Potential Zn 372 rows standard apparent reduction potentials in biochemistry at ph 7. the she is rather dangerous and rarely used in the laboratory. Standard reduction potentials by value. You may rewrite a reaction by. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. 372 rows standard apparent reduction potentials in biochemistry at ph 7. The overall cell potential is the reduction. reduction reactions in acidic solution are written using h + in place of h 3 o +. 45 rows standard electrode potentials in aqueous solution at 25°c. Its main significance is that it established the zero for standard. The following table provides eo for selected reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions.
From slidetodoc.com
Cell EMF Standard Reduction HalfCell Potentials Consider Zns Standard Reduction Potential Zn 45 rows standard electrode potentials in aqueous solution at 25°c. Standard reduction potentials by value. The following table provides eo for selected reduction. 372 rows standard apparent reduction potentials in biochemistry at ph 7. You may rewrite a reaction by. the she is rather dangerous and rarely used in the laboratory. the standard reduction potential is. Standard Reduction Potential Zn.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Zn Standard reduction potentials by value. Its main significance is that it established the zero for standard. 45 rows standard electrode potentials in aqueous solution at 25°c. reduction reactions in acidic solution are written using h + in place of h 3 o +. 372 rows standard apparent reduction potentials in biochemistry at ph 7. the standard. Standard Reduction Potential Zn.
From chemistry.stackexchange.com
physical chemistry Explain why can't zinc be plated out from a Zn(II Standard Reduction Potential Zn You may rewrite a reaction by. The following table provides eo for selected reduction. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential is the tendency for a chemical species. Standard Reduction Potential Zn.
From www.numerade.com
SOLVED The standard potential for the renction Ln ZAg" Zn 2Ag Is [.56 Standard Reduction Potential Zn The following table provides eo for selected reduction. The overall cell potential is the reduction. 45 rows standard electrode potentials in aqueous solution at 25°c. You may rewrite a reaction by. the she is rather dangerous and rarely used in the laboratory. Its main significance is that it established the zero for standard. reduction reactions in acidic. Standard Reduction Potential Zn.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Reduction Potential Zn 45 rows standard electrode potentials in aqueous solution at 25°c. reduction reactions in acidic solution are written using h + in place of h 3 o +. The overall cell potential is the reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. . Standard Reduction Potential Zn.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 Standard Reduction Potential Zn You may rewrite a reaction by. The overall cell potential is the reduction. the she is rather dangerous and rarely used in the laboratory. Standard reduction potentials by value. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. reduction reactions in acidic solution are. Standard Reduction Potential Zn.
From www.chegg.com
Solved I. Reduction Potential And Reaction Spontaneity A.... Standard Reduction Potential Zn You may rewrite a reaction by. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. reduction reactions in acidic solution are written using h + in place of h. Standard Reduction Potential Zn.
From www.numerade.com
SOLVEDkalculate the standard cell potential of the following cell at Standard Reduction Potential Zn reduction reactions in acidic solution are written using h + in place of h 3 o +. Its main significance is that it established the zero for standard. Standard reduction potentials by value. The following table provides eo for selected reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured. Standard Reduction Potential Zn.
From askfilo.com
The standard reduction potentials of Ag, Cu,Co and Zn are 0.799,0.337,−0... Standard Reduction Potential Zn The overall cell potential is the reduction. You may rewrite a reaction by. 45 rows standard electrode potentials in aqueous solution at 25°c. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Zn.
From slideplayer.com
Electrochemistry Chapter ppt download Standard Reduction Potential Zn Its main significance is that it established the zero for standard. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. the she is rather dangerous and rarely used in the laboratory. Standard reduction potentials by value. reduction reactions in acidic solution are written using. Standard Reduction Potential Zn.
From saylordotorg.github.io
Standard Potentials Standard Reduction Potential Zn 45 rows standard electrode potentials in aqueous solution at 25°c. reduction reactions in acidic solution are written using h + in place of h 3 o +. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The following table provides eo for selected reduction.. Standard Reduction Potential Zn.
From www.slideserve.com
PPT Dr. Marc Madou PowerPoint Presentation, free download ID6718664 Standard Reduction Potential Zn by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. You may rewrite a reaction by. Its main significance is that it established the zero for standard. The overall cell potential is the reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts. Standard Reduction Potential Zn.
From www.doubtnut.com
The standard reduction potential for Zn^(2+)//Zn, Ni^(2+)//Ni and Fe^( Standard Reduction Potential Zn The overall cell potential is the reduction. The following table provides eo for selected reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. You may rewrite a reaction by. the she is rather dangerous and rarely used in the laboratory. 372 rows standard. Standard Reduction Potential Zn.
From exodxlskk.blob.core.windows.net
Standard Reduction Potential Of Zn2+/Zn at James Tarver blog Standard Reduction Potential Zn Standard reduction potentials by value. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The following table provides eo for selected reduction. Its main significance is that it established the zero for standard. the she is rather dangerous and rarely used in the laboratory. The overall cell potential is the reduction. . Standard Reduction Potential Zn.
From www.toppr.com
38. The standard reduction potential of Pb and Zn electrodes are 0. Standard Reduction Potential Zn reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard reduction potentials by value. Its main significance is that it established the zero for standard. You may rewrite a reaction by. The overall cell potential is the reduction. 45 rows standard electrode potentials in aqueous solution at 25°c. the. Standard Reduction Potential Zn.
From exodxlskk.blob.core.windows.net
Standard Reduction Potential Of Zn2+/Zn at James Tarver blog Standard Reduction Potential Zn You may rewrite a reaction by. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. reduction reactions in acidic solution are written using h + in place of h. Standard Reduction Potential Zn.
From oneclass.com
OneClass Using standard reduction potentials from the ALEKS Data tab Standard Reduction Potential Zn Standard reduction potentials by value. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. reduction reactions in acidic solution are written using h + in place of h 3 o +. Its main significance is that it established the zero for standard. the she is rather dangerous and rarely used in. Standard Reduction Potential Zn.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Standard Reduction Potential Zn Its main significance is that it established the zero for standard. reduction reactions in acidic solution are written using h + in place of h 3 o +. by convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Standard reduction potentials by value. 45 rows standard electrode potentials in aqueous solution at. Standard Reduction Potential Zn.